Single-layer graphene is interesting as a flexible 2D material, with xy-dimensions variable up to a centimetre in length and a z-thickness of a single carbon atom. It conducts heat and electricity, has excellent mechanical strength, and is impermeable to gases except hydrogen gas. Its drawback: how to disperse it in a liquid. When you try to do this flexible sheets of graphene tend to stack as a result of attractive van der Waals interactions, making it virtually an impossible material to disperse as single sheets.
Labels are big business. A typical label has multiple layers: a topcoat for protection, the face stock, which contains the message in the form of text and/or images, a pressure-sensitive adhesive, and a release liner, which often has a release coating. The release liner and coating are only there to protect the label from sticking to things you do not wish it would stick to. You remove the liner when you wish to apply the label onto your substrate of choice, for example, a bottle containing a drink.
Imagine a label without a release liner and coating, imagine a label that could be activated at the moment you want it to stick to a substrate, a stick-on-demand linerless label.
BonLab has designed and developed a concept and prototype for a sustainable solution: a mesh reinforced pressure-sensitive adhesive for linerless label design. The idea was worked out by Emily Brogden and prof. dr. ir. Stefan Bon, in collaboration with UPM Raflatac Oy, a global supplier of label materials for branding and promotion, information and functional labelling (patent application: WO2023105120A1). The complete study, which was done at the University of Warwick, is now published in the new journal RSC Applied Polymers.
A mini-emulsion polymerization is a variation on the more conventional emulsion polymerization process in that in the ideal scenario latex particles are formed by monomer droplet nucleation. The monomer droplets are turned into polymer particles. The trick to achieve this is to shrink monomer emulsion droplets to sub-micrometer diameters. For this two ingredients are key, one is a lyophobe, a compound that dissolves in the monomer droplet but does not like to partition into the continuous phase, here water. Typically n-hexadecane is used. This compound suppresses coarsening, also called Ostwald ripening, of the droplets by providing an Osmotic counter pressure. The other essential ingredient is a surfactant which aids to stabilize the large combined surface area of the droplets and keeps then from colliding and fusing (colloidal stability).
The use of molecular surfactants, however, can have negative impacts when the polymer latex is used in formulations and applications as the surfactant can migrate. For example in a clear coating it could lead to uptake of water, causing the transparent coating to become opaque, a phenomenon known as water whitening.
When we synthesize polymer colloids by emulsion polymerization, molecular surfactants are often employed. These are required to keep the polymer latex particles dispersed in the water phase, so that they do not clump together, a phenomenon known as coagulation. Keeping polymer dispersions stable is especially important in end applications, such as waterborne coatings and adhesives.
A downside of the use of surfactant molecules is that they can desorb from the surface of the latex particles. This makes the particles colloidally unstable, and they coagulate. This can be disastrous in product formulations, such as water-based paints which have many components. Another downside of this mobility of the surfactant molecules is that they can migrate in the final coating, once applied on a substrate. This leads to deterioration of the properties of the coated film.
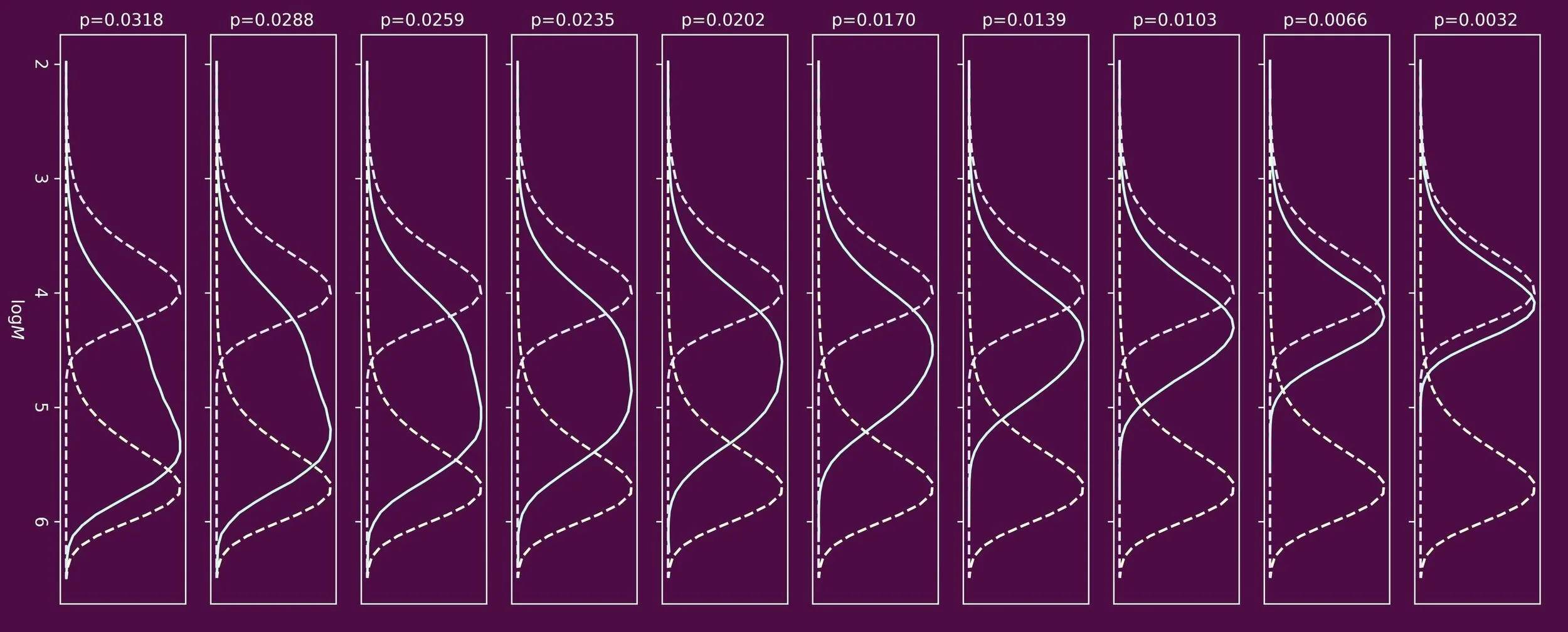
Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
Emulsion polymerization is of pivotal importance as a route to the fabrication of water-based synthetic polymer colloids. The product is often referred to as a polymer latex and plays a crucial role in a wide variety of applications spanning coatings (protective/decorative/automotive), adhesives (pressure sensitive/laminating/construction), paper and inks, gloves and condoms, carpets, non-wovens, leather, asphalt paving, redispersible powders, and as plastic material modifiers.
Since its discovery in the 1920s the emulsion polymerization process and its mechanistic understanding has evolved. Our most noticeable past contributions include the first reversible-deactivation nitroxide-mediated radical emulsion polymerization (Macromolecules 1997: DOI 10.1021/ma961003s), and the development and mechanistic understanding of Pickering mini-emulsion (Macromolecules 2005: DOI 10.1021/ma051070z) and emulsion polymerization processes (J. Am. Chem. Soc. 2008: DOI 10.1021/ja807242k). The latest on nano-silica stabilized Pickering Emulsion Polymerization from our lab can be found here.
One quest in emulsion polymerization technology that remains challenging and intriguing is control of the particle morphology. It is of importance as the architecture of the polymer colloid influences its behavioural properties when used in applications. We now report in ACS Nano an elegant innovation in the emulsion polymerization process which makes use of nanogels as stabilizers and allows us to fabricate Janus and patchy polymer colloids.
Call them plastics, polymers, elastomers, thermoplasts, thermosets, or macromolecules. What’s in the name? Despite the current negative press in view of considerable environmental concerns on how we deal with polymer materials post-use, it cannot be denied that polymers have been a catalyst in the evolution of human society in the 20st century, and continue to do so.
One of the synthetic pathways toward polymer molecules is free radical polymerization, a process known since the late 1800s and conceptually developed from the 1920s-1930s onwards. Since the 1980s it gradually became possible to tailor the chemical composition and chain architecture of a macromolecule. The process is called reversible deactivation radical polymerization (RDRP), also known as controlled or living radical polymerization. By grabbing control on how individual polymer chains are made, with the ability to control the sequencing of its building blocks, known as monomers, true man-made design of large functional molecules has become reality. This architectural control of polymer molecules allows for materials to be formulated with unprecedented physical and mechanical properties.
One interesting phenomenon is that when we carry out an RDRP reaction using a “living” polymer (a first block) dissolved in for example water and try to extend the macromolecule by growing a second block that does not dissolve in water, it is possible to arrange the blockcopolymer molecules by grouping them together into a variety of small (colloidal) structures dispersed in water. More interestingly, these assembled suprastructures have the ability to dynamically change shape throughout the polymerization process, for example to transform from spherical, to cylindrical, to vesicle type objects. This Polymerization Induced Self-Assembly process has been given the acronym PISA.
Emulsion polymerization is an important industrial production method to prepare latexes. Polymer latex particles are typically 40-1000 nm and dispersed in water. The polymer dispersions find application in wide ranges of products, such as coatings and adhesives, gloves and condoms, paper textiles and carpets, concrete reinforcement, and so on.
Conventional emulsion polymerization processes make use of molecular surfactants, which aids the polymerization reaction during which the particles are made and keeps the polymer colloids dispersed in water. We, and others, introduced Pickering emulsion polymerization a decade ago in which we replace common surfactants with inorganic nanoparticles.
In Pickering emulsion polymerization the polymer particles made are covered with an armor of the inorganic nanoparticles. This offers a nanocomposite colloid which may have intriguing properties and features not present in conventional "naked" polymer latexes.
To fully exploit this innovation in emulsion polymers, a mechanistic understanding of the polymerization process is essential. Current understanding is limited which restricts the use of the technique in the fabrication of more complex, multilayered colloids.
In the field of colloid science the ability to fabricate particles with a defined shape, other than a sphere, has gained attention. The reason is that anisotropy in shape and/or chemical composition can lead to interesting physical properties when these particles are dispersed in a liquid, or when they form part of a product formulation. We report an insight into the synthesis of silica-based “matchstick”-shaped colloidal particles, which are of interest in the area of self-propulsion on small length scales. The generation of aqueous emulsion droplets dispersed in an n-pentanol-rich continuous phase and their use as reaction centers allows for the fabrication of siliceous microparticles that exhibit anisotropy in both particle morphology, that is, a “matchstick” shape, and chemistry, that is, a transition-metal oxide-enriched head. We provide a series of kinetic studies to gain a mechanistic understanding and unravel the particle formation and growth processes. Additionally, we demonstrate the ability to select the aspect ratio of the “matchstick” particle in a straightforward manner.
The paper is recently published in Langmuir. DOI:10.1021/acs.langmuir.5b02645










Polymer latex particles, typically 50-600 nm in diameter, are used in many applications, such as paper manufacturing, water-based adhesives, printing, and coatings. Commonly, a water-based formulation that contains these polymer colloids is used, often together with other components, such as pigments for opacity and color, fillers, and rheology modifiers. Each of the polymer latex particles consists of many individual polymer chains. These water-based dispersions are applied onto a substrate as a droplet or a film, after which these systems are dried. Upon evaporation of water, the individual components will pack closely. When little water remains in between, a so-called capillary under-pressure facilitates tight packing, and if the polymer latex particles are soft, it deforms them. The last stage of the film formation process is when polymer chains from one latex particle now diffuse into a neighboring latex particle and the other way around. This process ensures that the dried film has good adhesive and mechanical properties.
Visualizing this drying and film formation process in real time would greatly help in understanding how the properties of a dried film come about. In our paper, published in the American Chemical Society’s journal Langmuir, we used TeraHertz Time-Domain Spectroscopy (THz-TDS) to map the water content spatially in real-time during the drying process.