Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
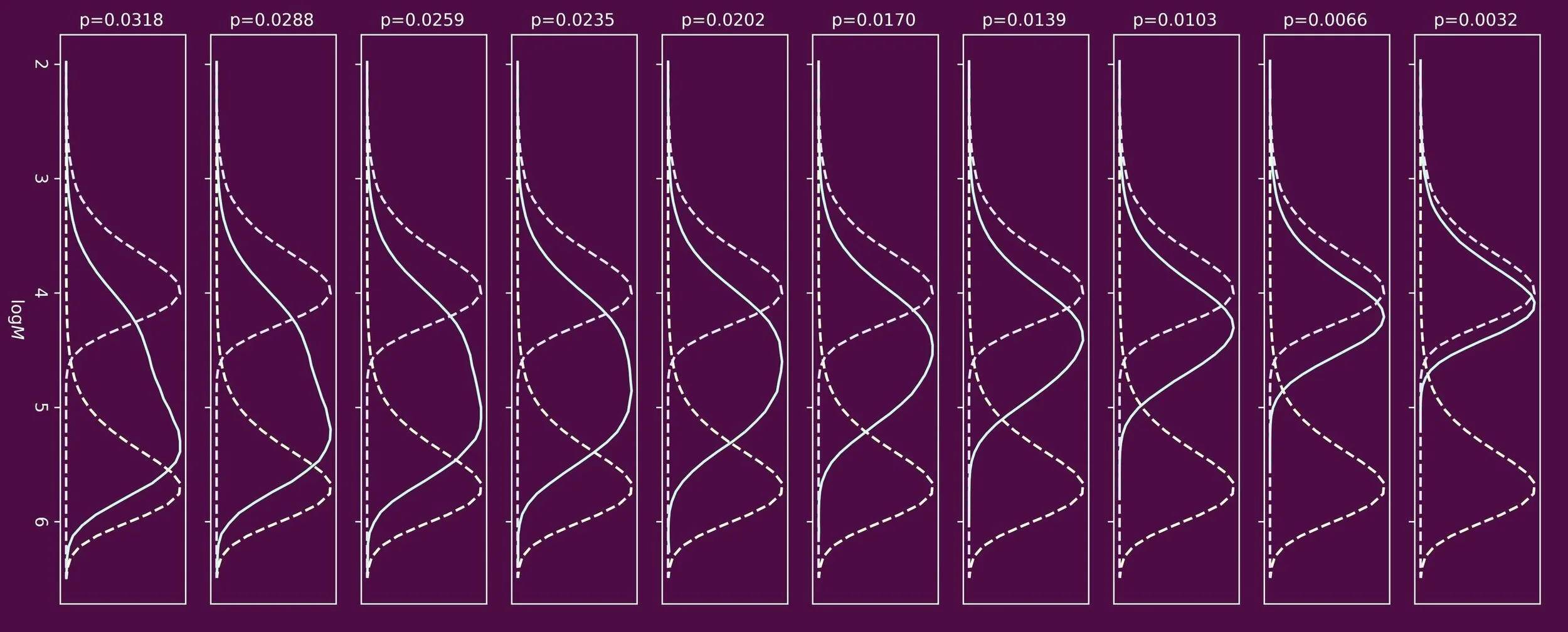
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
The most famous method to determine values for the chain transfer constant, Ctr, is referred to as the Mayo method. It is an excellent method, but over time its assumptions and boundary conditions have been eroded. The Mayo method is only valid if there is no marked composition drift, that is a drift in concentration ratio of chain transfer agent and monomer. In essence it makes use of the instantaneous molecular weight distribution, here in the form of the number average molecular weight. Whereas this is easy to achieve in experiments where Ctr < 1 by keeping monomer conversion low, this is less easy to do in cases where Ctr > 1.
In the same time period (1940s) Smith already reported a solution. Whereas elegant, in this method there is no direct link with the molecular weight distribution, which may sit uncomfortably.
In our paper entitled When Mayo falls short (Ctr ≫ 1): the use of cumulative chain length distribution data in the determination of chain transfer constants (Ctr) for radical polymerizations published in the RSC journal Polymer Chemistry we propose a new method. Here we make use of the cumulative molecular weight distribution, hereby taking into account composition drift.
The validity of our method is demonstrated using Monte Carlo simulations and its experimental usefulness is shown studying one of the most challenging systems of all: the free radical polymerization of vinyl acetate in presence of thiols as chain transfer agent. We are happy to report a value for the chain transfer constant of dodecanethiol of 223, at 333 K.
Prof. dr. ir. Stefan Bon says: “ I am delighted with the final version of the manuscript. The hard work by PhD student Matt Donald to carry out the experiment and his dedication in bringing together wet science with Monte Carlo simulations paid off. We both hope that our method will be embraced, tested, and used by others. “
Details on the paper can be found here: